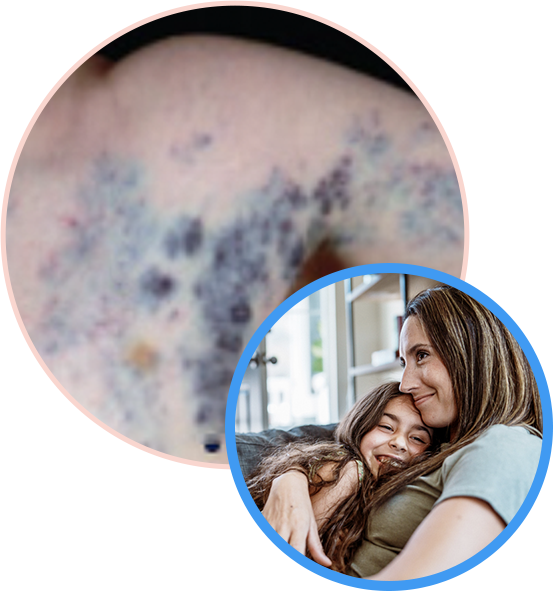
All Participants Receive Treatment
The TOIVA Trial is investigating whether you may see a prolonged reduction of veins that are pushing through the surface of the skin.
All trial participants will receive treatment at no cost with the study medication for the duration of the study, as well as for an optional extension period afterward.
Compensation and reimbursement for travel to a study site for the study participant and a caregiver are provided by the study sponsor.
Additional information can be obtained from the national clinical study registry, Clinicaltrials.gov (Click here for more information: NCT06653842).
Why Should I Participate in the TOIVA Trial?
- The study medication is a gel that the patient can easily apply to their skin on their own once a day at home.
- You will be treated by a doctor at a nationally renowned medical center.
- All appointments, including travel and lodging for the participant and one caregiver are provided at
no cost,
and you will also receive
compensation
for taking part in the study.
- Additional medication may be provided to you for an optional treatment extension period after the study concludes.
- You will be helping to find an effective treatment for venous malformations and VM disease for patients like you.

See if you are Eligible
Clinically Diagnosed VM Visible at the Skin Surface
Patients Must be at least 6 Years Old or Older
A Confirmed Genetic Test of Blood or Tissue is a PLUS
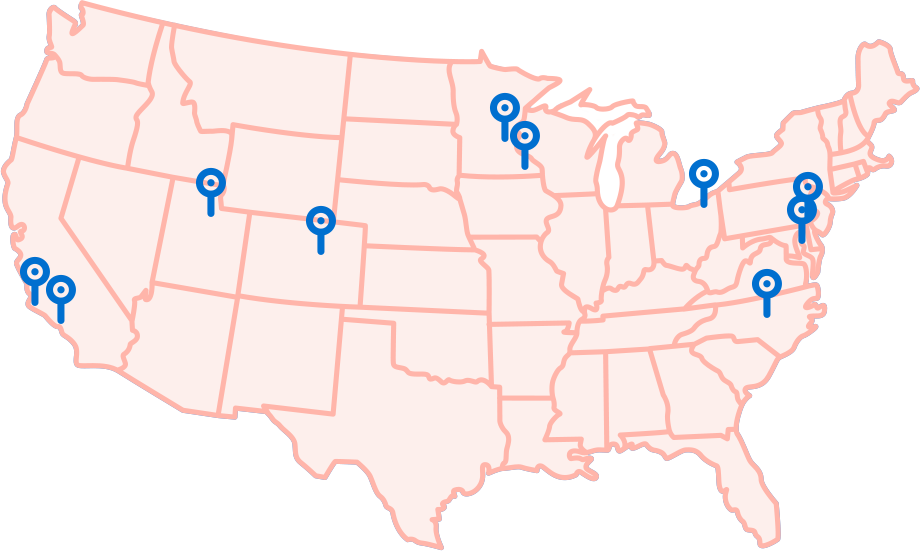
Where is the TOIVA Trial Taking Place?
Check the map below to see if there is a trial location close to you.
Compensation + Reimbursement for Travel and Lodging is available for the Participant and 1 Caregiver from anywhere within the U.S. or Canada.
University of California Irvine/ Children's Hospital of Orange County
Irvine, CA
Now Recruiting
Stanford University
Palo Alto, CA
Now Recruiting
Colorado Children's Hospital
Aurora, CO
Now Recruiting
Johns Hopkins
Baltimore, MD
Now Recruiting
Minnesota Clinical Study Center /
Associated Skincare Specialists
New Brighton, MN
Now Recruiting
Mayo Clinic
Rochester, MN
Now Recruiting
University of North Carolina
Chapel Hill, NC
Now Recruiting
Cleveland Clinic
Cleveland, OH
Now Recruiting
Children's Hospital of Philadelphia
Philadelphia, PA
Now Recruiting
University of Utah Health
Salt Lake City, UT
Now Recruiting
Frequently Asked Questions
What is a clinical trial?
A clinical trial, sometimes called a “study,” is the investigation of a medicine in a new way. Sometimes the medicine has been studied and approved for other diseases. Clinical trials in the United States are closely regulated by the Food & Drug Administration (FDA). Study participants and their doctors are provided with information specific to the trial to help them make a decision about whether the study is right for them.
What is the medicine being tested?
All patients in the TOIVA Trial will treat themselves at home with a new gel formulation of an approved drug (rapamycin, also known as sirolimus). The gel will be rubbed onto the skin once a day by the participant, or for very young children, by their caregiver.
What are Cutaneous Venous Malformations?
Venous Malformation (VM) is a type of vascular anomaly where a person’s veins grow in an abnormal way, sometimes called VM disease. A cutaneous Venous Malformation (or cVM) is typically purplish or blue in color and is visible just beneath the skin, sometimes protruding or bulging out of the skin. A cutaneous VM can be delicate and painful, and can sometimes cause problems, especially if it is located near joints or other organs. In some patients the skin is also tender and fragile, and friction from clothes or bedding can lead to chafing and bleeding.
How much will participants be compensated for participating in the study?
Compensation is offered to offset patient burden and should not be considered the reason for participation. If you or your child is a good match for the study, the details related to the study stipend will be provided to you.
What if I don't see a location near me?
The study sponsor will pay travel expenses to a study location that is convenient for both the participant and one caregiver.




